DRY CHARGE BATTERY
We offer vast range of Dry Charge Batteries or Conventional Batteries. Our Batteries are designed to have filler caps and vent tubes. Not all vehicles require SMF batteries and Dry Charge/Conventional Batteries offer good performance and longevity but at a lower price point.
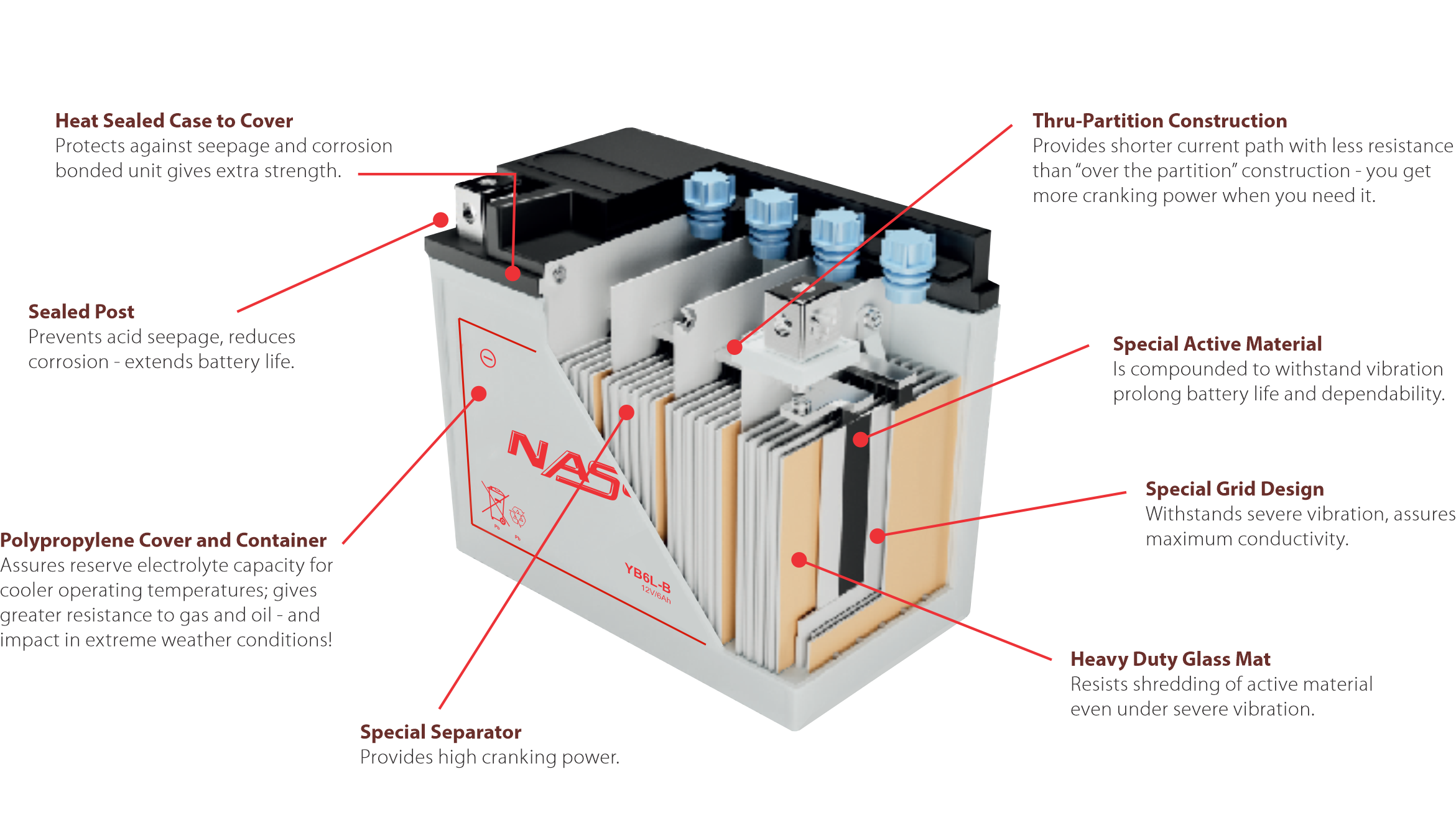
Important aspects of the battery include sealed posts to resist corrosion, tough polypropylene covers, containers and heat sealed construction for a strong, bonded unit. In addition, design features include special separators and through-partition construction.
Our batteries have more cranking power (up to 30%) for their physical size than other standard Conventional battery. The plate surface area in the battery is increased by the use of thin, high-tech separators that make room for extra plates within each cell.
Features:
- 20% – 30% more starting power than conventional batteries
- More plates per cell
- High cranking under different climatic conditions
- Anti Sulfation Protection given for consistent performance
- Battery Containers designed for ultra-low vibrations, high durability and longer service life
- Low self-discharge means longer ideal life even after adding electrolyte
| Item | Model | Voltage(V) | Capacity(Ah) | Terminal | L(mm) | W(mm) | H(mm) |
|---|---|---|---|---|---|---|---|
| 1 | 6N4-2A | 6 | 4 | + ,- | 71 | 71 | 96 |
| 2 | 6N4-2A-2 | 6 | 4 | + ,- | 71 | 71 | 96 |
| 3 | 6N4-2A-4 | 6 | 4 | + ,- | 71 | 71 | 96 |
| 4 | 6N4-2A-7 | 6 | 4 | + ,- | 71 | 71 | 96 |
| 5 | 6N6-3B-1 | 6 | 4 | -,+ | 99 | 57 | 111 |
| 6 | 12N5-3B | 12 | 5 | -,+ | 120 | 61 | 130 |
| 7 | 12N5-4B | 12 | 5 | +,- | 120 | 61 | 130 |
| 8 | YB5L-B | 12 | 5 | -,+ | 120 | 61 | 130 |
| 9 | 12N6-3B | 12 | 6 | -,+ | 137 | 72 | 95 |
| 10 | YB6L-B | 12 | 6 | -,+ | 137 | 72 | 95 |
| 11 | 12N6.5-3B | 12 | 6.5 | -,+ | 138 | 73 | 107 |
| 12 | YB6.5L-B | 12 | 6.5 | -,+ | 138 | 73 | 107 |
| 13 | 12N7A-3A | 12 | 7 | -,+ | 150 | 60 | 130 |
| 14 | 12N7B-3A | 12 | 7 | -,+ | 150 | 60 | 130 |
| 15 | 12N7A-4A | 12 | 7 | +,- | 150 | 60 | 130 |
| 16 | 12N7B-4A | 12 | 7 | +,- | 150 | 60 | 130 |
| 17 | YB7B-B | 12 | 7 | +,- | 150 | 60 | 130 |
| 18 | 12N7-3A | 12 | 7 | -,+ | 135 | 75 | 133 |
| 19 | 12N7-3B | 12 | 7 | -,+ | 135 | 75 | 133 |
| 20 | 12N7-4A | 12 | 7 | +,- | 135 | 75 | 133 |
| 21 | 12N7-4B | 12 | 7 | +,- | 135 | 75 | 133 |
| 22 | YB7L-B | 12 | 7 | -,+ | 135 | 75 | 133 |
| 23 | 12N9-3A | 12 | 9 | -,+ | 135 | 75 | 139 |
| 24 | 12N9-3B | 12 | 9 | -,+ | 135 | 75 | 139 |
| 25 | 12N9-4B | 12 | 9 | +,- | 135 | 75 | 139 |
| 26 | 12N9-4B-1 | 12 | 9 | +,- | 135 | 75 | 139 |
| 27 | YB9-B | 12 | 9 | +,- | 135 | 75 | 139 |
| 28 | 12N10-3B | 12 | 10 | -,+ | 134 | 90 | 145 |
| 29 | YB10L-B | 12 | 10 | -,+ | 134 | 90 | 145 |
| 30 | 12N12A-4A-1 | 12 | 12 | +,- | 136 | 76 | 160 |
| 31 | YB12A-A | 12 | 12 | -,+ | 134 | 76 | 160 |
| 32 | YB12AL-A | 12 | 12 | -,+ | 134 | 76 | 160 |
| 33 | 12N14-3A | 12 | 14 | -,+ | 136 | 90 | 164 |
| 34 | 12N14-3B | 12 | 14 | -,+ | 136 | 90 | 164 |
| 35 | YB14-A2 | 12 | 14 | +,- | 136 | 90 | 164 |
| 36 | YB14L-A2 | 12 | 14 | -,+ | 136 | 90 | 164 |
| 37 | YB2.5L-C | 12 | 2.5 | -,+ | 80 | 70 | 105 |
| 38 | YB2.5L-C2 | 12 | 2.5 | -,+ | 80 | 70 | 105 |
| 39 | 12N3-3A | 12 | 3 | -,+ | 98 | 56 | 110 |
| 40 | YB3L-A | 12 | 3 | -,+ | 98 | 56 | 110 |
| 41 | YB3L-B | 12 | 3 | -,+ | 98 | 56 | 110 |
| 42 | YB3L-C | 12 | 3 | -,+ | 98 | 56 | 110 |
| 43 | YB4L-B | 12 | 4 | -,+ | 120 | 70 | 92 |
| 44 | YB16L-B | 12 | 16 | -,+ | 75 | 100 | 155 |
| 45 | 12N16-3B | 12 | 16 | -,+ | 75 | 100 | 155 |
| 46 | 51913 | 12 | 19 | -,+ | 185 | 82 | 170 |
| 47 | YB16AL-A2 | 12 | 16 | -,+ | 205 | 70 | 162 |
HOW TO CHARGE A BATTERY
Dry Charge/Conventional Battery Charging Procedure:
- Place it on a level surface
- Remove the yellow filled caps, placing them safely on one side
- Remove the sealing caps from the vent, never replace this after the battery has been filled with electrolyte as it may cause the battery to rupture
- Fill each cell with electrolyte to the fill level as indicated on the battery case (Always wear protective eyewear and gloves when working with electrolytes)
- The electrolyte should have specific gravity of 1.265 and be between 62 – 86 ° F
- Leave the battery for a minimum of 30 minutes and gently tap occasionally on the case to remove any bubbles trapped between the p

- If after 30 minutes the electrolyte level has fallen, fill it to the upper fill level as indicated on the battery case
- Replace the filled caps loosely and begin to charge the battery at 1/10 of its rated capacity for 3-5 hours. Charging at higher rate could damage the battery
- Do not connect or disconnect the battery while the charger is switched on as this may cause sparks that could ignite the hydrogen gas emitted from the cells during charging
- Monitor the electrolyte level during charging and top up the fill line as necessary
- When charging is complete, turn off the charger and disconnect it from the battery
- Push or screw down the yellow filler caps. Make sure not to over – tighten them
- Clean off any spilled electrolyte with water and baking soda solution
- Allow the battery to stand of at least 30 minutes
- Load test the battery at 3 times its ampere hour rating for 15 seconds or use an automatic battery tester to determine the battery condition and then check the voltage
- Voltage should be minimum 12.4V on a 12V battery
- Battery is then ready to be fitted
- If the voltage reading is below 12.4V or the battery fails the automatic battery test, loosen the filler caps and repeat the charging and test cycle
TECHNICAL FEATURES
Technical Features
- Sealed Construction
The unique construction and sealing technique ensures no electrolyte leakage from case or terminals. - Electrolyte Suspension System
All batteries utilize Nasoki’s unique electrolyte suspension system incorporating a micro fine glass mat to retain the maximum amount of electrolyte in the cells. The electrolyte is retained in the separator material and there is no free electrolyte to escape from the cells. No gels or other contaminants are added. - Recombination Technology
The design of Nasoki’s batteries incorporates the very latest oxygen recombination technology to effectively eliminate the need for watering during normal use. - Low Maintenance Operation
Due to the perfectly sealed construction and the recombination of gasses within the cell, the battery is almost maintenance free. - Terminals
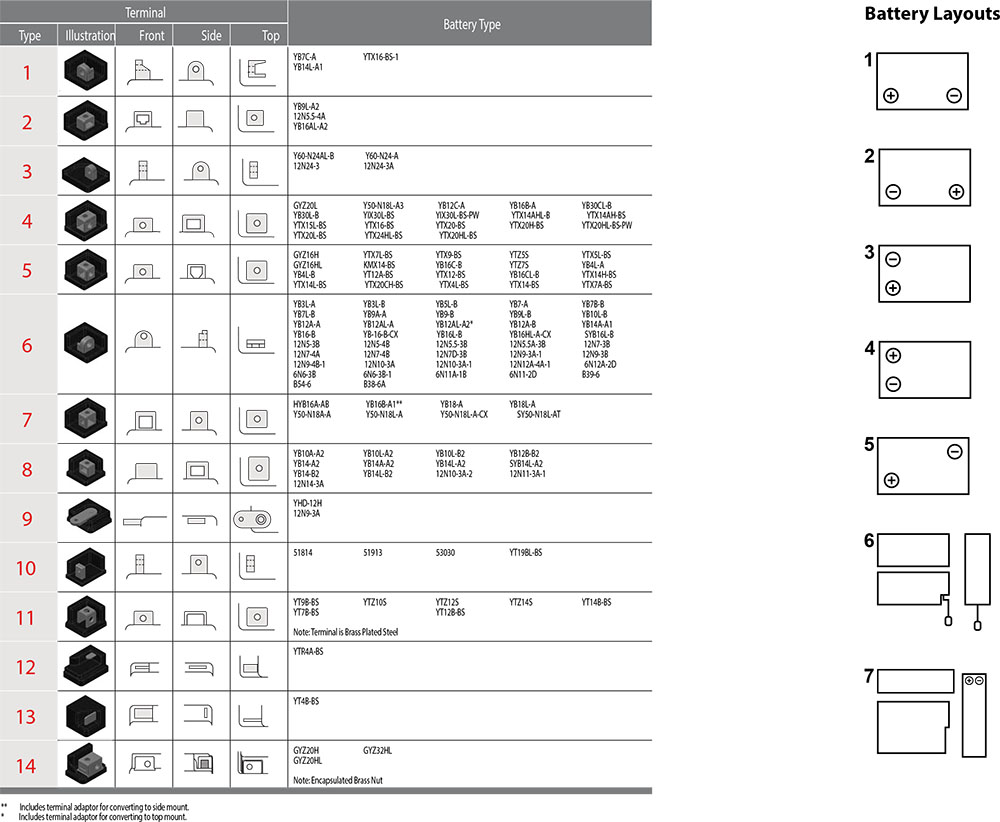
Batteries are manufactured using a range of terminals which vary in size and type. Please refer to details as shown below:
Terminal Configurations
Terminal shapes vary from one battery to another. By identifying the correct replacement battery from the listing in website, you are assured of the proper terminal configuration.
BATTERY SAFETY

Here’s an 8-point list that’ll help keep those hazards under control:
- Absolutely no smoking, sparks or open flames around batteries. Batteries can produce hydrogen and oxygen; if they ignite the battery can rupture.
- On conventional batteries, loosen vent caps when charging and ventilate the entire charging area. A build-up of hydrogen and oxygen levels in the battery or in the room where it’s being charged can create a hazard.
- If a battery feels hot to touch during charging, stop charging and allow it to cool before resuming. Heat damages the plates, and a battery that’s too hot can rupture.
- Never put the red sealing cap back on the battery once you take it off. If you do, gases trapped inside can explode. Make sure the vent tube isn’t kinked or blocked, for the same reason.
- Connect the charger to battery properly: positive to positive, negative to negative. Unplug the charger or turn it off before you disconnect the lead: that cuts down on the chance of sparks.
- Always wear eye protection, protective gloves and protective clothing.
- Clean up acid spills immediately, using a water and baking soda solution to neutralize (1 lb. baking soda in 1 gal. water).
- Make sure acid container is clearly marked and the work area is well lighted.
If sulfuric acid is swallowed or splashed in the eyes, take immediate action. While the diluted sulfuric acid used as electrolyte can burn the skin, this type of injury is generally less serious. Sulfuric acid in the eyes can cause blindness. Serious internal injuries or death can result from ingesting sulfuric acid.
Antidotes
- External – Flush with water.
- Internal – Drink large quantities of milk or water, followed by milk of magnesia, vegetable oil or beaten eggs. Call a poison control center or doctor immediately.
- Eyes – Flush for several minutes with water, get immediate medical attention.
Points To Remember
- Ventilate battery charging area.
- Charging gives off gases – no smoking, sparks or flames.
- Safety glasses or face shields protect against eye damage.
- Acid swallowed or in the eyes requires immediate antidotes and medical care.
- All safety considerations are important… review them frequently.
Battery Testing Devices
How much of a charge does a battery have? There are two easy and reliable ways to find out:
- A hydrometer, which comes in floating ball and calibrated float types, or
- A voltmeter (or multimeter, which gives DC voltage readings).
Which is the best?
If you’re choosing between two hydrometers, opt for the calibrated float type. It gives you an exact specific gravity reading (that is, the density of the electrolyte compared to water), that’s much more accurate than floating balls. A voltmeter or multimeter can be used where a hydrometer can’t. Most sealed or low maintenance batteries have to be tested with a voltmeter.
Battery testing requires a voltmeter that can measure DC voltage. Remember to always connect a voltmeter parallel to the circuit being tested, observing polarity; otherwise, the pointer will travel in the wrong direction. It’s a good idea to periodically check a voltmeter against another one of known accuracy.
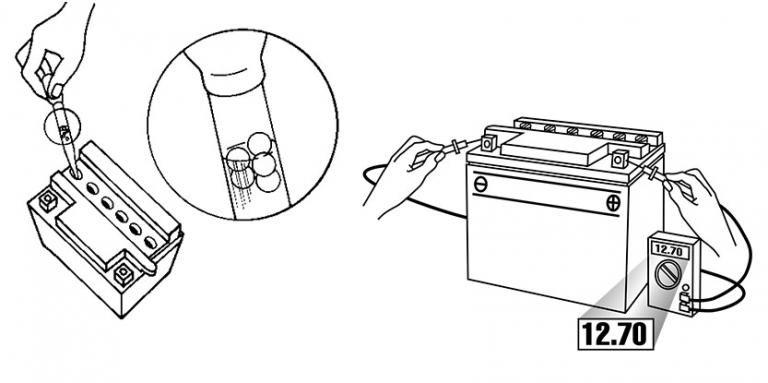
BATTERY TESTING
There are two types of battery tests: Unloaded and Loaded. An Unloaded Test is made on a battery without discharging current. It’s simplest and most commonly used. And if you need a precise reading, loaded testing is the answer. It’s more accurate.
Unloading Testing
Check charge condition using either a hydrometer or voltmeter. With a voltmeter, voltage readings appear instantly to show the state of charge. Remember to hook the positive lead to the battery’s positive terminal, and the negative lead to the negative terminal.
A hydrometer measures the specific gravity of each cell. The specific gravity tells the degree of charge: generally, a specific gravity of about 1.265 to 1.280 indicates a full charge. A reading of 1.230 to 1.260 indicates the battery should be charged before testing. The chart below shows the charge level as measured by syringe float hydrometer, digital voltmeter and five – ball hydrometer.
Methods of Checking Battery Condition
| State of Charge | Syringe Hydrometer | Digital Voltmeter | 5-Ball Hydrometer |
|---|---|---|---|
| 100% Charged w/Sulfate Stop | 1.280 | 12.80v | 5 Balls Floating |
| 100% Charged | 1.265 | 12.60v | 4 Balls Floating |
| 75% Charged | 1.210 | 12.40v | 3 Balls Floating |
| 50% Charged | 1.160 | 12.10v | 2 Balls Floating |
| 25% Charged | 1.120 | 11.90v | 1 Balls Floating |
| 0% Charged | less than 1.100 | less than 11.80v | 0 Balls Floating |
A battery’s specific gravity changes with temperature. Ideally, readings should be taken at 77° f. Is it really going to matter if you’re off a couple of degrees one way or another? Probably not. If you’re working somewhere that’s uncomfortably hot or cold, it’s time to use the old conversion factors: add 0.001 to the specific gravity reading for each 3°F above 77°F or subtract 0.001 from the specific gravity reading for each 3°F below 77°F. Cell voltage can be found by adding .84 to the specific gravity.
Note: Too, that Nasoki’s “Sulfate Stop”, a chemical additive that increases battery life by drastically reducing sulfate buildup, changes the specific gravity reading; they’ll be higher than any ordinary batteries.

Embracing innovation and advanced technology, Nasoki offers the best batteries in the market. Performance, reliability, and power are an assurance with us.
Contact Us
- Eastman Auto & Power Ltd – Automotive Division 572, Udyog vihar, Phase-V, Gurugram – 122016. Haryana, India
- Phone: +(91) - (124) - 4627900
- eaplenquiry@eastmanglobal.com
Nasoki 2020 | All Rights Reserved ©2020 Nasoki Battery.